United states pharmacopeia (usp), code of federal regulations (cfr) and aoac international methods for antibiotic potency testing

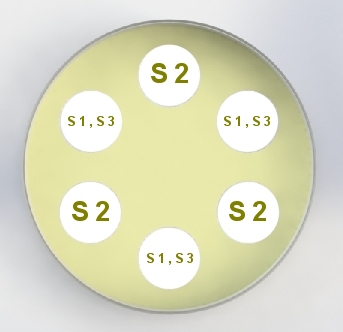
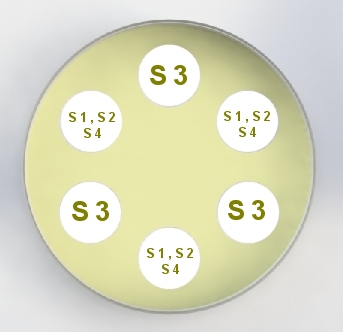
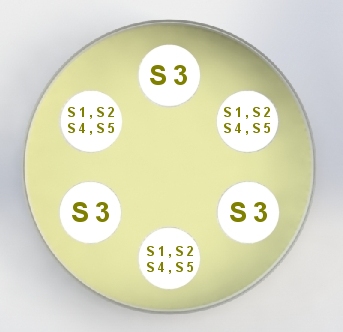
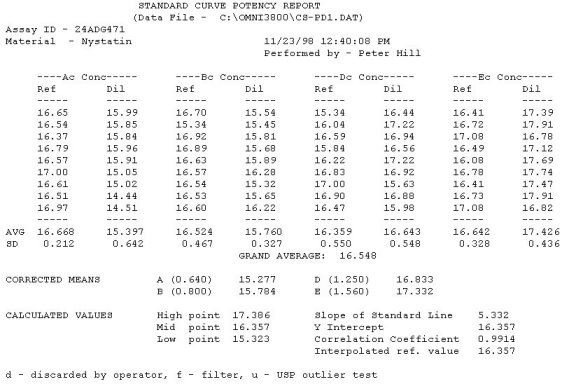
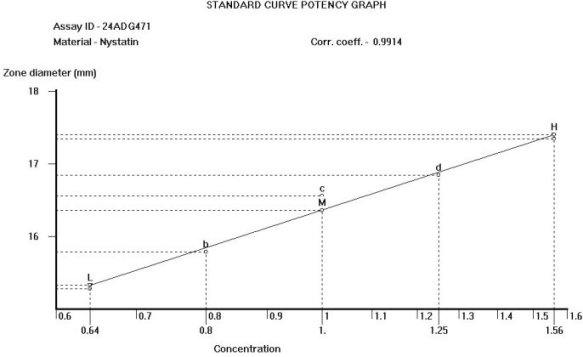
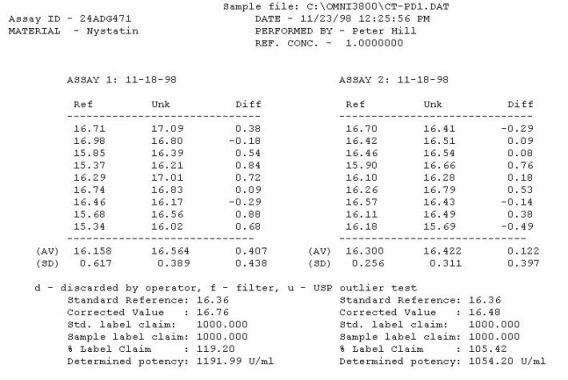
United States Pharmacopeia (USP), Code of Federal Regulations (CFR) and AOAC International methods for Antibiotic Potency Testing The OMNICON USP Zones of Inhibition software performs all calculations in accordance to the procedures defined in the Code of Federal Regulations Standard (CFR) 436.105 as well as in the Section of Design and Analysis of Biological Assays <111>, Chapter of Biological Tests, United States Pharmacopoeia (USP). The software calculates the mean and standard deviation values, excludes zones based on the USP Outlier Test and draws the Zones of Inhibition potency line graph for the Standard Analysis. It also calculates the differences between the reference assay and the sample test assay; obtain the mean and standard deviation of these differences, calculate the label claim percentage and the determined potency value for the Trial Analysis, or the Trial analysis results based on weight and dilution. Plate Reading 21 CFR, Part 11, Electronic Records and Signatures Assay Layout
Assay Reports |


 GENTAUR Europe BVBA
GENTAUR Europe BVBA GENTAUR France SARL
GENTAUR France SARL GENTAUR GmbH
GENTAUR GmbH  GENTAUR Ltd.
GENTAUR Ltd.  GENTAUR Poland Sp. z o.o.
GENTAUR Poland Sp. z o.o.  GENTAUR Nederland BV
GENTAUR Nederland BV GENTAUR SRL IVA IT03841300167
GENTAUR SRL IVA IT03841300167 GENTAUR Spain
GENTAUR Spain GENTAUR BULGARIA
GENTAUR BULGARIA